Document administration is A vital ingredient of top quality administration software, guaranteeing that every one documents are handled in accordance with expectations, tips, and laws.
The following facts really should be recorded at the time Just about every motion is taken (the day should be mentioned and the person dependable need to be Plainly determined by signature or Digital password):
It really is A vital A part of GMP to maintain precise documents, and during an audit it can help convey the message that treatments are now being adopted. Furthermore, it demonstrates that the procedures are regarded and are under Manage.
System Qualification: Approach Qualification is intended to discover a results of the method which can determine the potential to breed business producing output. Throughout this method, all crucial quality parameter is taken under consideration to make sure merchandise good quality.
Audit stories are documents that detail the conclusions from internal or external evaluations performed to assess compliance with applicable necessities. In pharmaceutical companies, audit experiences show compliance with inner, consumer, and regulatory specifications.
SimplerQMS presents life science QMS application with sturdy document administration capabilities, enabling pharmaceutical companies to competently handle and Regulate documents and records during their lifecycle.
Adhering to are classified as the posts linked to pharmaceutical quality assurance and good quality administration method for pharmaceutical industry All those are handy for new in addition to knowledgeable pharmaceutical specialists. This web site is updated frequently hence, do not forget to visit once more.
All associates provide the accountability of guaranteeing that each one GMP routines are carried out according to the official SOPs; any deviations in method are reported for their supervisor and therefore are adequately documented.
● Any worker should not be permitted to signal for one more member of staff members Unless of course delegated. Signatures ought to under no circumstances be solid.
These data should be numbered with a unique batch or identification range and dated and signed when issued. In continuous production, the products code together with the day and time can function the unique identifier until eventually the ultimate selection is allocated.
What about the digitalization of SOPs and information adhering to a ZERO PAPER coverage? check here In case we deliver documents only having an IT system is it necessary to help keep the Uncooked knowledge if we retain a scanned duplicate? ReplyDelete
Some companies might also involve supplemental qualifications or training in quality administration systems or According to regulatory necessities.
Validation and high-quality assurance will go hand in hand, guaranteeing the standard for the products and solutions. The current post offers an introduction and common overview on system validation of pharmaceutical production process In particular pill production
The details outlined in these documents could override Instructions provided in other degree documents. (By way of example: the organization’s documentation SOP may possibly condition that numbers be rounded off here to three significant figures; the batch history, Alternatively, might point out that each one figures be expressed in scientific notation. Hence, Directions in level four documents, which might be precise to a specific method, can overrule the instruction talked about in amount 3 documents, which can be common in mother nature. The document hierarchy pyramid is A technique of organizing a business’s documents.
 Emilio Estevez Then & Now!
Emilio Estevez Then & Now!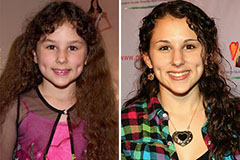 Hallie Eisenberg Then & Now!
Hallie Eisenberg Then & Now! Alexa Vega Then & Now!
Alexa Vega Then & Now! Seth Green Then & Now!
Seth Green Then & Now! Tiffany Trump Then & Now!
Tiffany Trump Then & Now!